Existence of Multiple Critical Cooling Rates which Generate Different Types of Monolithic Metallic Glass
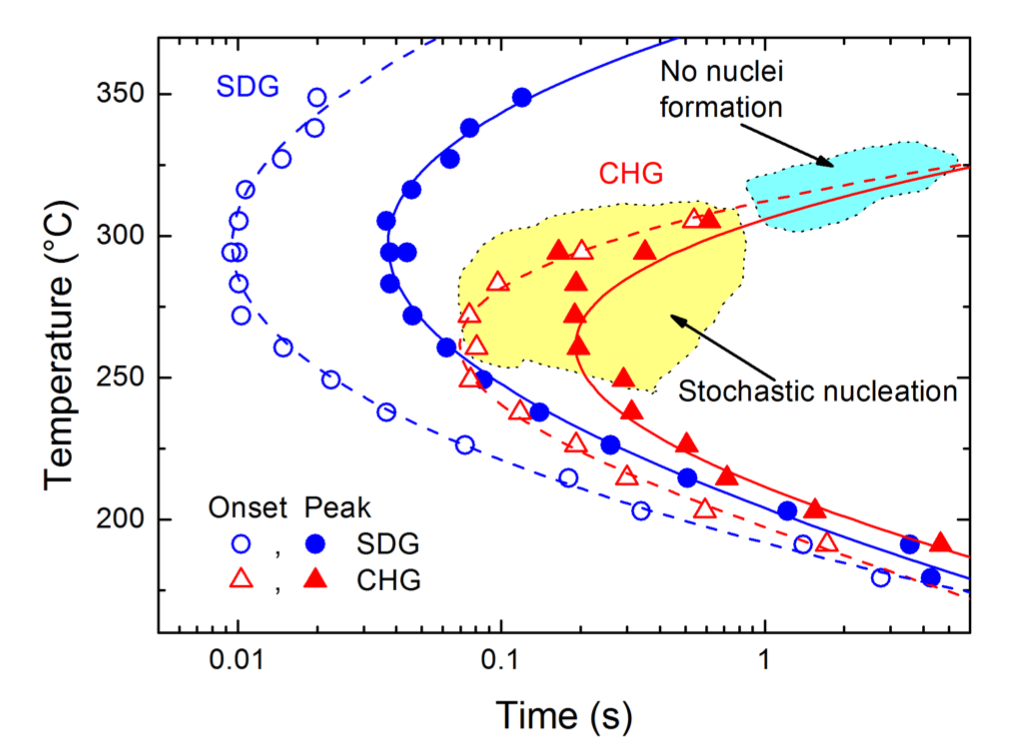
In a recent Nat. Comm. publication, Jörg F. Löffler from the Laboratory of Metal Physics and Technology (LMPT) and his colleague Jürgen Schawe from Mettler Toledo show that metallic glasses generally reveal multiple critical cooling rates that produce different types of monolithic glass, which the authors termed “self-doped glass” (SDG) and “chemically homogeneous glass” (CHG). The CHG reveals a tendency towards stochastic nucleation, which underlines the novelty of this glass state.
The competition between glass formation and nucleation is an important materials science phenomenon and occurs in various classes of metastable materials with different types of bonding. In a recent Nat. Comm. publication, Jörg F. Löffler of LMPT with a colleague from Mettler Toledo deployed a newly developed ultrafast scanning calorimeter (prototype of a Flash DSC2+) to determine the thermophysical properties of metallic glass-forming systems at ultrafast rates. The authors document that monolithic glasses of the same kind can have distinctly different short-range order with pronounced property variations. They thus classified these glasses into two categories and termed them “self-doped glass” (SDG), which forms quenched-in nuclei upon cooling, and “chemically homogeneous glass” (CHG).
The authors also demonstrate that the occurrence of such glass types is a general phenomenon in metallic glass formation. In contrast to SDG, CHG reveals a tendency towards stochastic nucleation, which highlights the novelty of this glass state. Since both types of glasses may also form in molecular systems, the authors expect this phenomenon to be also of high relevance to other areas such as pharmaceuticals and biology. Their distinction of different kinds of monolithic glass has important consequences on glass processing and may also help to develop a more generalized glass theory.
- Existence of multiple critical cooling rates which generate different types of monolithic metallic glass, Jürgen E.K. Schawe and Jörg F. Löffler, Nature Comm. 10, 1337 (2019). external pageDOI:10.1038/s41467-018-07930-3call_made (2019). article supplementary information
- Laboratory of Metal Physics and Technology
- external pageMettler-Toledo Analyticscall_made