Biocorrosion Zoomed In: Evidence for Dealloying of Nanometric Intermetallic Particles in Magnesium Alloys
In a recent Advanced Materials publication, researchers at the Laboratory of Metal Physics and Technology (LMPT) present TEM experiments designed to follow the active nano-corrosion processes of biodegradable Mg alloys over periods ranging from seconds to days. They find that cathodically polarized dealloying of intermetallic nanoprecipitates governs their electrochemical reactivity. This presents a fundamentally new concept for active materials such as Mg alloys.
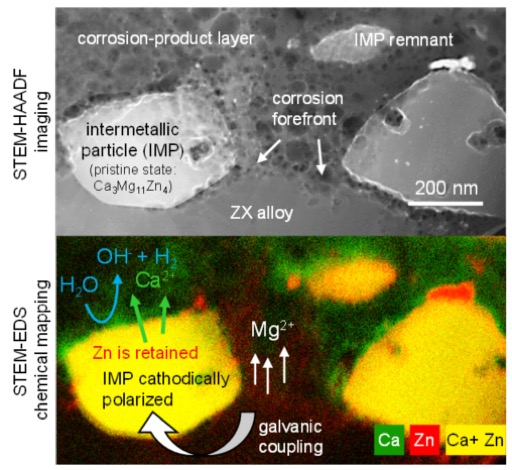
Extensive research into the use of magnesium (Mg) alloys in temporary biomedical implants has been ongoing for several years, and the first commercial products involving Mg are already being deployed in clinics. As illustrated by in vitro and in vivo animal and clinical trials, bioresorbable Mg alloys have significant advantages over both bioinert metals such as stainless steel or titanium, and biodegradable polymers. Nevertheless, an in-depth understanding of the fundamental electrochemical mechanisms with which Mg alloys degrade in complex physiological environments has remained fragmentary until now.
To remedy this situation the authors of the Advanced Materials study determined the nano- and microscopic chemical changes associated with Mg dissolution by deploying a correlative transmission electron microscopy (TEM)-based approach that combines quasi in situ and ex situ biocorrosion experiments. On this basis they were able to document a mechanism of dealloying that occurs during the corrosion of nanometric precipitates. The latter are generally present in high-performance Mg alloys, and specifically in the bioresorbable MgZnCa alloys investigated in the study. The new mechanism, termed “cathodically polarized dealloying,” makes it possible to rationalize the evolution of elemental decomposition and thus to predict Mg’s prolonged degradation behavior.
The dealloying mechanism identified in the Advanced Materials study represents a new development in the understanding of Mg corrosion, because dealloying generates a gradual change in Mg’s microstructural chemistry and thus dynamically changes its electrochemical activity. This observation of dynamic effects thus augurs a change in mindset, because up to now the research community has considered the material’s microstructure to remain unchanged during its dissolution – generating large errors in predicting Mg degradation. The mechanism identified appears to be universal, as it is expected to also occur in other Mg systems and generally across active materials that contain intermetallic precipitates.
- Biocorrosion Zoomed In: Evidence for Dealloying of Nanometric Intermetallic Particles in Magnesium Alloys, M. Cihova, P. Schmutz, R. Schäublin, J. F. Löffler, Adv. Mater. 1903080 (2019) 1-10. external pageDOI:10.1002/adma.201903080call_made
- Laboratory of Metal Physics and Technology
- external pageEmpa, Laboratory for Joining Technologies and Corrosioncall_made
